To learn more, you can visit our free guide on
How to Prepare for the GAMSAT ®
. In addition, if you're yet to familiarise yourself with the different sections of the GAMSAT ® Exam, you can find further free GAMSAT ® Preparation Materials below:
Free GAMSAT Preparation Materials
-
GAMSAT ® Preparation Materials: Section 1
Guide:
How to Prepare for GAMSAT ® Section 1
An overview of what to expect in Section 1 (Reasoning in Humanities and Social Sciences) of the GAMSAT ®
Exam and how to prepare: Includes study tips, MCQ tricks and a reading list.
-
GAMSAT ® Preparation Materials: Section 2
Guide:
How to Prepare for GAMSAT ® Section 2
An overview of what to expect in Section 2 (Written Communication) of the GAMSAT ®
Exam and how to prepare: Learn how to build up an ideas bank and how to structure your writing.
Essay Topics:
Free GAMSAT ® Section 2 Quote Generator
Get over 90 free essay topics for Section 2 of the GAMSAT ®
Exam
Guide:
GAMSAT Example Essays
Download our GAMSAT ®
Essay Writing guide with 3 marked GAMSAT ® example essays of varying qualities.
-
GAMSAT ® Preparation Materials: Section 3
Guide:
How to Prepare for GAMSAT ® Section 3
An overview of what to expect in Section 3 (Reasoning in Biological and Physical Sciences) of the GAMSAT ®
Exam and how to prepare. Get a topic list of everything you need to know for Biology, Chemistry and Physics.
Guide:
How to Prepare for GAMSAT ® Biology
Get even further details and specific tips for the Biology component of Section 3 of the GAMSAT ®
Exam
Guide:
How to Prepare for GAMSAT ® Chemistry
Further advice and information specific to GAMSAT ®
Section 3 Chemistry - Get a detailed breakdown of various topics.
Guide:
How to Prepare for GAMSAT ® Physics
Not sure about the value of preparing for GAMSAT ®
Physics? Think again - The Physics component of Section 3 can be a key separator of student performance, get further details on how to prepare.
It's important to remember that the GAMSAT ® exam that tests your reasoning and problem solving skills rather than your recall of specific content - As such, it's important that you avoid trying to study for it as if it were a knowledge-based exam. The greater focus should lie in learning to apply that knowledge and using it in a problem-solving setting - i.e. Problem Based Learning (you can learn more
here
). This is most effectively done by completing GAMSAT ® Practice Questions.
GradReady GAMSAT
®
Free Trial
To get even more free MCQs, make sure you sign up for our GAMSAT Free Trial which includes 50 free MCQs from our Intelligent MCQ Bank as well as a wealth of other free resources.
Start Your GAMSAT ®
Preparation Today!
Our Intelligent GAMSAT ®
MCQ Bank utilises cutting edge web education technology in performance tracking and topic targeting.
The 7000+ MCQs are categorised into 43 subtopics - our advanced MCQ system not only tracks your performance in each subtopic, it also allows you to create sets of MCQs based on subtopics of your choosing – meaning you can focus on what you need to. In addition, you are able to set the difficulty of the questions, allowing you to tailor the MCQs to your own ability. Fully Worked Solutions are provided for all MCQs.
By signing up to our GAMSAT ®
Free Trial, you will also get access to GradReady’s Online Exam System, which mimics the official ACER GAMSAT ® exam in detail, allowing you to familiarise yourself with the online delivery of the exam before test date. You can simulate the full GAMSAT ®
experience with the associated time pressures, or complete the exam at your own pace if you prefer. You will also get access to worked solutions, extra supplementary resources, performance tracking, percentile reporting, and more!
We are the only GAMSAT ®
Preparation Provider with our own proprietary online system - Unlike other providers who simply purchase a 3rd Party System, we’ve gone to the trouble of creating our online system to allow you to track your performance, quickly identifying your weaknesses and pointing you to the most relevant materials and even tutor assistance. Our online algorithmic-assisted resources are designed to provide you with a targeted experience and makes learning into a science.
In addition, because it is a proprietary system, we are able to constantly make updates in line with student feedback to ensure that students are getting the best learning experience possible. Our newly updated Online Learning Management systems means that we are the only GAMSAT ®
Preparation Provider with a mobile-friendly experience - You can now utilise our Intelligent MCQ Bank whenever and wherever your phone goes.
Free GAMSAT
®
Practice Questions
If you’re looking for some free GAMSAT ®
Practice Questions, you can find several examples below, separated according to their different Sections:
GAMSAT ® Section 1 Practice Question
The following is a poem titled
A Bird Came Down The Walk
by Emily Dickinson.
A bird came down the walk:
He did not know I saw;
He bit an angle-worm in halves
And ate the fellow, raw.
And then he drank a dew
From a convenient grass,
And then hopped sidewise to the wall
To let a beetle pass.
He glanced with rapid eyes
That hurried all abroad,
They looked like frightened beads, I thought;
He stirred his velvet head
Like one in danger; cautious,
I offered him a crumb,
And he unrolled his feathers
And rowed him softer home
Than oars divide the ocean,
Too silver for a seam,
Or butterflies, off banks of noon,
Leap, splashless, as they swim.
1. What theme is most evident in A Bird Came Down The Walk?
- a) Cruelty
- b) Benevolence
- c) Nature
- d) Humanity
2. In the final two stanzas, the bird is described as flying away. The depiction that the bird's flight "rowed him softer..." than oars dividing the ocean implies:
- a) Natural beauty surpasses man-made beauty
- b) Passage by flight is more inspiring than passage by sea
- c) There is a stark, superficial, contrast between animals and humans
- d) Nature supersedes nurture
GAMSAT ® Section 2 Practice Question
Theme: Knowledge
-
A little knowledge is a dangerous thing. So is a lot.
(Albert Einstein)
-
Some people still think knowledge is power.
(Chuck Palahniuk, Lullaby)
-
The power of human thought grows exponentially with the number of minds that share that thought.
(Dan Brown, The Lost Symbol)
-
The thing that's important to know is that you never know. You're always sort of feeling your way.
(Diane Arbus, Diane Arbus Revelations)
-
Any fool can know. The point is to understand.
(Albert Einstein)
You can find more free GAMSAT ®
Essay Topics and a Free GAMSAT ®
Essay Quote Generator at our guide here:
GAMSAT ® Section 2 Essays: How to Prepare
You can find some free example essays here:
Free GAMSAT ® Example Essay
GAMSAT ® Section 3 Biology Practice Question
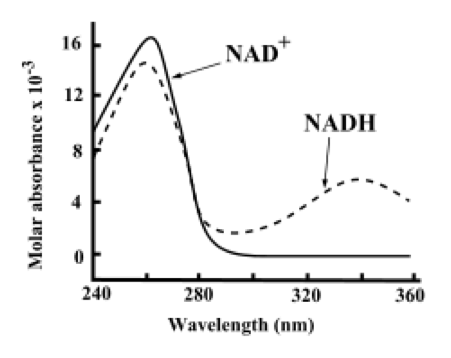
The diagram above shows the absorbance of NAD+ and NADH at various wavelengths of light. As NAD+ is a dinucleotide it absorbs, like all nucleotides, strongly in the 260-280 nm range. It can also be seen that the protonated form exhibits a modified absorbance spectra.
An important biochemical measure of the health of a cell is known as the redox state and is the ratio of NAD+ to NADH. A healthy cell has a redox state of around 700. This high ratio makes oxidative reactions favourable and thus enables oxidative phosphorylation, or the aerobic formation of ATP.
To do this, reference samples of known concentrations of NAD+ and NADH are made up and measured in a spectrophotometer. The unknown samples are then measured and compared to the reference samples to determine the quantity of NAD+ or NADH in the sample.
A molecular biologist has a number of samples that they wish to test the redox state of. For each question select the correct answer using the knowledge provided in this section
1. What wavelength would be most appropriate for determining the concentration of NAD
+
?
-
a) 240 nm
-
b) 260 nm
-
c) 340 nm
-
d) 300 nm
2. What wavelength would be most appropriate for determining the concentration of NADH when discriminating against NAD
+
concentration?
-
a) 240 nm
-
b) 260 nm
-
c) 340 nm
-
d) 300 nm
3. What redox state would most likely be found in a cell that was overwhelmed with the work of metabolizing alcohol?
-
a) NAD+/NADH greater than 700
-
b) NAD+/ NADH less than 700
-
c) NAD+/ NADH equal to 700
-
d) Not enough information to determine
GAMSAT ® Section 3 Chemistry Practice Question
The increased emission of carbon dioxide into the atmosphere leads to a decrease in the pH of ocean water due to a chemical equilibrium between carbon dioxide (CO
2
) and carbonic acid (H
2
CO
3
) which can then contribute protons into the ocean:
CO
2
+ H
2
O ↔ H
2
CO
3
H
2
CO
3
↔ H
+
+ HCO
3
-
HCO
3
-
↔ H
+
+ CO
3
2-
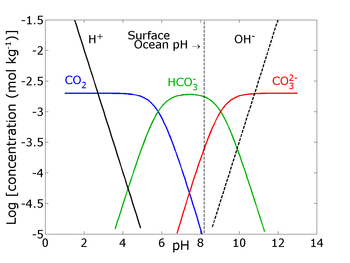
The relative abundance of the above species at a given pH is represented on a Bjerrum Plot.
1. In 1751 the ocean pH was 8.25, but it is expected by 2100 that [H+] in the ocean will increase by 2.5 fold. Which of the following indicates the expected ocean pH in 2100?
-
a) 10.75
-
b) 7.85
-
c) 7.25
-
d) 5.75
2. The initial [H
+
] in 1751 was 5.62 * 10
-9
M, and Ca(OH)
2
is a strong base that is expected to dissociate completely. How much Ca(OH)
2
(1.0 M) would be required to return a 4 L water sample from the year 2100 back to the acidities of year 1751?
-
a) 11.2 nanolitres
-
b) 17.0 nanolitres
-
c) 28.1 nanolitres
-
d) 56.2 nanolitres
3. Oceanic species such as corals and plankton have calcium carbonate (CaCO
3
) skeletal structures that require the levels of CO
3
2-
to be saturated in ocean waters. What effect would an increase in CO
2
emissions have on these species?
-
a) The dissolving of their skeletons
-
b) The forming of carbonate aggregates on their skeletons
-
c) A thriving environment for skeletal formation
-
d) No obvious effect
GAMSAT ® Section 3 Physics Practice Question
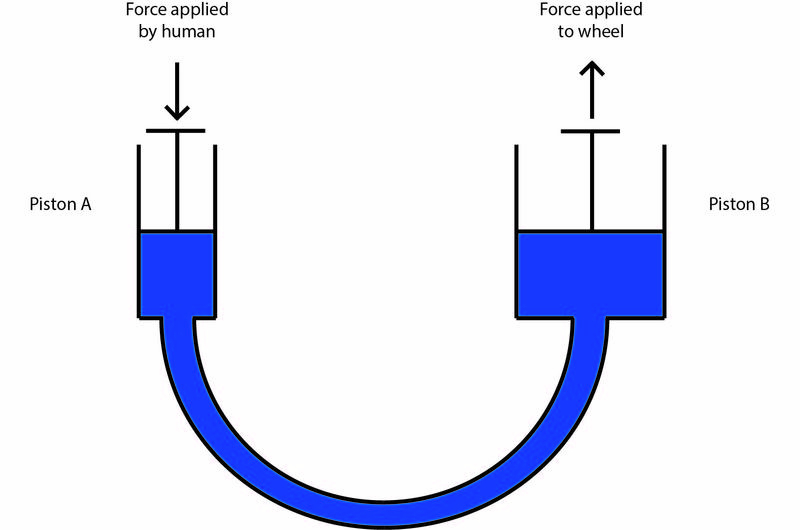
Hydraulic force multiplication is one of the mechanisms that allow the force applied to a car’s brake pedal by a person's foot to be multiplied to a level that can stop a car quickly. A very simplified diagram is provided below.
Piston A and B are both cylindrical and are at the same height. The fluid used, in blue, is water. The diameter of Piston A is 10 cm.
1. How much pressure would the person be applying to the water if they push down on the brake pedal with 200 N of force?
-
a) 10/π kPa
-
b) 2/π kPa
-
c) 20/π kPa
-
d) 80/π kPa
2. It is known that a fluid’s density increases as temperature increases and decreases as the temperature decreases. If the efficiency of the hydraulic braking system is defined as how effectively the forces are multiplied, what happens to the braking system efficiency on a hot day?
-
a) Pressure increases, brake efficiency increases
-
b) Pressure increases, Brake efficiency decreases
-
c) Pressure decreases, Brake efficiency remains constant
-
d) Pressure increases, brake efficiency remains constant
If you find the practice MCQs above useful, make sure you sign up for our GAMSAT ®
Free Trial which includes 50 free MCQs from our Intelligent MCQ Bank. You will also get access to a GAMSAT ® practice test which mimics the official ACER GAMSAT ®
exam in detail, allowing you to familiarise yourself with the online delivery of the exam before test date.
Start Your GAMSAT ®
Preparation Today!
GAMSAT Practice Question Answers
GAMSAT Section 1 Practice Question Answers
1. C is correct.
Dickinson repeatedly compares and contrasts the cruelty/fear ("...bit an angle-worm in halves" depicting the predator-prey relationship between bird and worm) and beauty ("...he unrolled his feathers, And rowed him softer home") of nature. Students may interpret the poem to represent the other themes, however these are either not suggested (Answers 1 and 2) or a minor theme (Answer 4).
2. A is correct.
As the comparative 'softer' is used, it suggests that the natural sight of a bird flying is more beautiful than boat oars that create gentle ripples in the water. This implies natural beauty surpasses man-made beauty, as oars and boats are man-made. Use of enjambment (the continuation of a sentence without a pause beyond the end of a line, couplet, or stanza) creates a relaxed tone.
GAMSAT Section 3 Biology Practice Question Answers
1. B is correct.
260nm is the answer as this is in the range light is most strongly absorbed by NAD
+
, which assists its measurement by a spectrophotometer
2. C is correct.
At 340nm, there is the greatest amount of difference between the standard molar absorbance of NAD
+
and NADH, and this would be the most appropriate point to measure the two comparatively.
3. B is correct.
In the stem, it is indicated that '
a healthy cell has a redox state of around 700. This high ratio makes oxidative reactions favourable and thus enables oxidative phosphorylation, or the aerobic formation of ATP
'.
We can infer that a cell overwhelmed with the work of metabolizing alcohol is less healthy, and therefore the ratio would be less than 700.
The enzymes used to metabolise ethanol (ADH and ALDH2) reduce NAD
+
to NADH, and therefore the cellular NAD
+
/ NADH redox ratio is lowered as a consequence of ethanol metabolism.
GAMSAT Section 3 Chemistry Practice Question Answers
1. B is correct.
Students should be familiar with pH being a logarithmic scale with a change in 1 pH unit corresponding to a [H+] change of a factor of 10. Therefore, for an acidification with a [H+] factor change of 2.5 the pH is expected to decrease, but by less than 1 pH unit. The only answer that fulfills this criteria is B.
2. B is correct.
Calculate difference in proton concentration by determining concentration in 2100 and taking away initial concentration.
In 1751: [H
+
] = 5.62 * 10
-9
M (mol L
-1
)
Multiply by 2.5 to get 2100 levels: [H
+
] = 14.1 * 10
-9
mol L
-1
Difference in [H
+
] (2100 levels - 1751 levels) = 8.48 * 10
-9
mol L
-1
Determine amount of H
+
to be neutralised in a 4 L sample (multiply difference by 4 L):
4 L sample = 33.9 * 10
-9
mol to be neutralised
Each Ca(OH)
2
provides two hydroxides, therefore we require half this amount of Ca(OH)
2
:
n(Ca(OH)
2
) = 17.0 * 10
-9
mol
Concentration is 1.0 M (mol L
-1
), therefore answer is 17.0 nanolitres (17.0 * 10
-9
L).
3. A is correct.
Of the extra carbon dioxide added into the oceans, some remains as dissolved carbon dioxide, while the rest contributes towards making additional carbonic acid and additional bicarbonate as in the equilibria. This also increases the concentration of hydrogen ions, and the percentage increase in hydrogen is larger than the percentage increase in bicarbonate, creating an imbalance in the reaction HCO
3
−
↔ CO
3
2−
+ H
+
. To maintain chemical equilibrium, some of the carbonate ions already in the ocean combine with some of the hydrogen ions to make further bicarbonate. Thus the ocean's concentration of carbonate ions is reduced, creating an imbalance in the reaction Ca
2+
+ CO
3
2-
↔ CaCO
3
, and making the dissolution of formed CaCO
3
structures more likely. In other words, the CO32- concentration drops, so that the ocean is no longer saturated and CO
3
2-
dissolves from the coral. This can be seen on the Bjerrum plot - as pH drops, the concentration of CO
3
2-
drops too.
GAMSAT Section 3 Physics Practice Question Answers
1. D is correct.
Using the formula P=F/A and keeping in mind that the
radius
of Piston A is 5 cm ,
Remember that Pa (pascals) is the equivalent of N/m
2
2. D is correct.
On a hot day, the density of the water increases.
Using the formula P = ρ X g X h it is clear that if the density (ρ) increases, then the pressure will increase.
However, this pressure increase is applied to both pistons. While this might increase the force applied to the water in Piston A, it increases the force applied to Piston B by the same proportion. In other words, the
ratio
of forces between the pistons remains the same, so the brake efficiency remains the same.
What types of questions are in the GAMSAT ®
Exam?
Section 1: GAMSAT ®
Reasoning in Humanities and Social Sciences
is comprised of solely multiple-choice questions, and focuses mostly on the candidate’s ability to interpret and understand various ideas which may arise from different social and cultural contexts. There will be a number of modalities presented here ranging from argumentative writings and academic essays, to poems and fictional stories. Visual stimuli will also be included, such as satirical newspaper cartoons. Emphasised through these stems will be the need for candidates to understand both explicit and implicit nuances, employ rationalisation to come to various conclusions, and make discriminations and judgements.
Section 2: GAMSAT ®
Written Communication
will assess the candidate’s ability to take a number of thematic statements offered as prompts, and create 2 essays based off these aforementioned prompts. Of particular importance here is the candidate’s ability to clearly articulate their thoughts, whilst offering up a thought-provoking response to the prompts, which often relate to socio-cultural or personal issues.
Section 3: GAMSAT ®
Reasoning in Biological and Physical Sciences
will include questions which cover a range of disciplines within science; namely, Chemistry, Biology and Physics. Chemistry will comprise 40% of the total Section III paper; Biology 40%, and Physics 20%. The expected standard of both Biology and Chemistry is to a first-year university level, whereas the expected standard for Physics is Year 12. This section focuses heavily on problem solving and will present graphs, tables and diagrams to the candidate for interpretation.
How many questions are in the GAMSAT ®
Exam?
Section 2: Written Communication is on a different day than the other two sections of the GAMSAT ® and will offer the candidate a number of essay prompts for a total of 2 questions corresponding to 2 essays, both of which must be completed within 65 minutes. GAMSAT ® Section 1 and 3 will occur around 3 weeks after the Written Communication section. There will be 62 multiple-choice questions (MCQs) in total for Section 1: Reasoning in Humanities and Social Sciences, which are to be completed within 100 minutes of test time. Lastly, Section 3: Reasoning in Biological and Physical Sciences will consist of 75 MCQs with a time limit of 150 for all questions. All MCQs in both Sections I and II will have 4 options to choose from.
How many GAMSAT ®
Exam practice questions should I do to get a good score?
Unfortunately there is no definite number of practice questions that you can do to guarantee a good or even passable score, especially given individual variation in terms of strengths and weaknesses.
However as a benchmark, 1 full practice test per week may be a good goal, whether this is timed or untimed. The key here is to focus on quality over quantity, ensuring you understand not only why the correct answers are correct, but why the incorrect answers are incorrect. In doing so, it becomes easier to identify areas of weakness and where you may need to undertake further study; for example, a bridging course or external textbooks. Regular, focused practice is the key to improving your knowledge and GAMSAT ®
exam results
as opposed to a lengthy test session sans review.
In addition to this full practice test per week, it would also be recommended to write at least 2 full essays, timed or untimed, per week. This not only assists in breaking up the relative monotony of Section I and III’s MCQs, but allows practice on a range of essay topics, some of which may be more difficult for individual candidates.
Ultimately, whilst there is no set number of questions or essays to go through per week for success in GAMSAT®, the key considerations remain the same: regular, consistent practice with focused reviews to identify weaknesses. As test day comes closer, it would also be recommended to graduate from untimed tests and essays to timed tests and essays, to best simulate the testing environment and time constraints.
Free GAMSAT Practice Test
To see where you’re at, and what you need to work on when it comes to your GAMSAT® preparation, have a go taking our GAMSAT ®
Free Trial
practice test.
Taking practice tests for the GAMSAT ® examination is crucial to your preparation. A practice test allows you to try things out and see where you are at and which concepts you might be struggling with or strong in.
Most importantly, practice tests allow self-reflection on the key aspects of both general test-taking abilities and those specific to the GAMSAT ®
:
Improve Your GAMSAT Time Management
Taking a
GAMSAT ® free practice test
allows you to gauge how quickly you are answering questions in sections 1 and 3. Many students struggle to finish these sections on time, and resort to guessing a significant portion of questions at the end of the time. Therefore, even simply finishing all questions on time gives you a significant edge over other students.
Doing a GAMSAT ® practice test online allows you to see whether you are completing Qs on time, or spending too long on individual questions. It allows you to gauge how long a set of Qs takes as opposed to individual Qs, and start to get a sense of how to allocate time between large and smaller stems.
In Section 2 Written Communication, many students fail to complete two essays in the time-frame allocated. If you take a practice test, you can get a sense of what length and detail is reasonable to aim for for yourself, and challenge yourself to balance quality/quantity. You also get a sense of the bank of ideas you have at your disposal and whether you need to develop more knowledge and material to imbue your essays with.
However, in order to gain this benefit from a practice test, the formal GAMSAT ® time restrictions should be followed. Check out our blog article for more tips on
how to manage time in the GAMSAT ® exam.
Get Familiar with GAMSAT Question Style
Understanding the format of each section and question styles is important. With Sections 1 and 3, you may want to read the stem first, or read the questions first, so finding what works for you early in preparation is key.
It is important to see how GAMSAT ® asks you to apply science concepts. While you may be studying endless amounts of chemistry, physics and biology, without knowing how questions ask you to apply such information, you will not fulfill your potential in the exam.
If you come from a non-science background, you may find our guide to
preparing for the GAMSAT ® with a non-science background
useful.
Gauge Your GAMSAT Subject Knowledge
Most relevant to section 3, taking GAMSAT ® practice tests allows you to gauge in which topics your knowledge may be lacking. This helps you to direct your time towards studying subjects/topics which are going to take you to the next level, rather than constantly going over things you may already be competent at. This is especially helpful if you are
preparing for the GAMSAT ® with a non-science background.
GradReady’s GAMSAT ®
Free Trial includes Online Exams which are integrated into the same intelligent MCQ system that tracks your performance across 43 subtopics - Take our Diagnostic Test and easily identify your strengths and weaknesses!
Sign up to test our industry-leading online learning technology for yourself:
Start Your GAMSAT ®
Preparation Today!
Further Free GAMSAT ®
Preparation Materials
-
The most comprehensive library of free GAMSAT Preparation materials available.
-
Covers everything you need to know about your GAMSAT ®
Results - How the scoring works, result release dates and even GAMSAT ® score cutoffs.
-
A breakdown of how to approach study effectively and how to set up a GAMSAT ® study schedule
-
An overview of what to expect in Section 1 of the GAMSAT ®
Exam and how to prepare.
-
An overview of what to expect in Section 2 of the GAMSAT ®
Exam, how to prepare and how to perfect your essay technique.
-
An overview of what to expect in Section 3 of the GAMSAT ®
Exam and how to prepare for each of the topics - Biology, Chemistry, & Physics.
-
Get even further details and specific tips for the Biology component of Section 3 of the GAMSAT ®
Exam
-
Further advice and information specific to GAMSAT ®
Section 3 Chemistry - Get a detailed breakdown of various topics.
-
Not sure about the value of preparing for GAMSAT ®
Physics? Think again - The Physics component of Section 3 can be a key separator of student performance, get further details on how to prepare.
Start Your GAMSAT ®
Preparation Today!
GradReady GAMSAT ® Preparation Courses
|
Course
|
Key Features
|
Description
|
|
GradReady GAMSAT ® Online Courses
|
|
Online (Essentials)
|
7000+ Intelligent MCQ Bank
1200+ page Textbook
8 Online Practice Exams
2 Marked Essays with Personal Feedback
5 Mock Exams
|
Designed for students who are unable to make our attendance classes, this course gives you the essentials to succeed.
|
|
Online (Comprehensive)
|
8 Marked Essays with Personal Feedback
15 Online Practice Exams
GetClarity: Tutor Assistance when you need it on whatever you need it on
|
All the features of our Online (Essentials) Course plus more Marked Essays, Practice Exams, and on-demand Tutor Assistance with our GetClarity system
|
|
GradReady GAMSAT ® Attendance Courses
|
|
Attendance (Comprehensive)
|
15 Day Course - 75+ hours of Learning
10 Marked Essays with Personal Feedback
GetClarity: Tutor Assistance when you need it on whatever you need it on
5 Mock Exams and Subsequent Review
Complimentary Additional Round of Virtual PBLs
|
Our most popular course. Includes all the live classes as well as access to our Online (Comprehensive) resources
|
|
Attendance (Complete Care)
|
15 Day Course - 75+ hours of Learning
12 x 1 hour Private Tutoring sessions
5 Mock Exams and Subsequent Review
Complimentary Additional Round of Virtual PBLs
|
Everything you need to succeed – includes private tutoring sessions in addition to the Attendance (Comprehensive) Package
|
|
Attendance (Success Assured)
|
15 Day Course - 75+ hours of Learning
36 x 1 hour Private Tutoring sessions
Personal GradReady Success Leader
5 Mock Exams and Subsequent Review
2 Complimentary Re-Enrolments (each valid for 1 year)
Complimentary InterviewReady Course Enrolment
|
Our most comprehensive course - valid for up to 3 years. You will be assigned a personal tutor (GradReady Success Leader) who will be supporting you throughout your journey
|
To learn more about our GAMSAT ® Preparation courses and compare their different components, view an in depth comparison here:
GradReady Course Comparison
If you’re after a single feature, such as our Textbook, or access to our 7000+ Intelligent MCQ Bank, or if you want to customise your preparation, you can do so here:
GradReady GAMSAT ® Custom Course.
At GradReady, we pride ourselves on providing students with the
Best Results
at the
Best Value:
-
Our team is consistently reinvesting in our internal operational technology to ensure that we're constantly improving our efficiency and productivity. The end result for our students is that we stand head and shoulders above our competition in the comprehensiveness of the tools we offer and the effectiveness of our teachings –
all at the best value
.
-
We believe in a data-driven approach: using student performance data to fine tune our practice questions, study content and teaching styles has allowed us to achieve
unparalleled results
for our students.
-
We are the only provider with Statistically Significant Proven Results – our students achieved an average improvement of
20+ Percentile Points, 12+ years in a row,
with an average improvement of 25 Percentile Points for the May 2020 GAMSAT ® exam.
-
We achieve these results through our interactive teaching style and adaptive online learning technologies. Our classes are capped at 21 students, and taught by a specialist tutor for each subject, tutors who are themselves Medical Students who have sat the GAMSAT ®.
- Our online systems make learning into a science and we are the only provider with a proprietary online system that uses algorithmic-assisted, targeted learning. Unlike other providers who purchase a 3rd Party System, the targeted system that we've developed tracks your performance, quickly identifying your weaknesses and pointing you to the most relevant materials and even tutor assistance.
To learn more about our courses and compare us to the competition, visit:
GradReady GAMSAT ® Preparation Courses